Correct Option(c)Cl>F>Br>I
Explanation:
As we go down the group in periodic table, atomic size increases, force of attraction for the added electron decreases. Thus, the added electron becomes loosely bound. Hence, electron gain enthalpy decreases down a group.
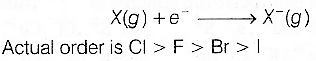
The fact that fluorine has a less electron gain enthalpy than chlorine seems to be due to the relatively greater the effectiveness of 2p-electron in the small F-atom to repel the additional electron entering the atom than 3p-electrons in the larger Cl-atom.