The `OH` group exerts `+M` (or + R) effect on the ring under the influence of attacking electrophile.
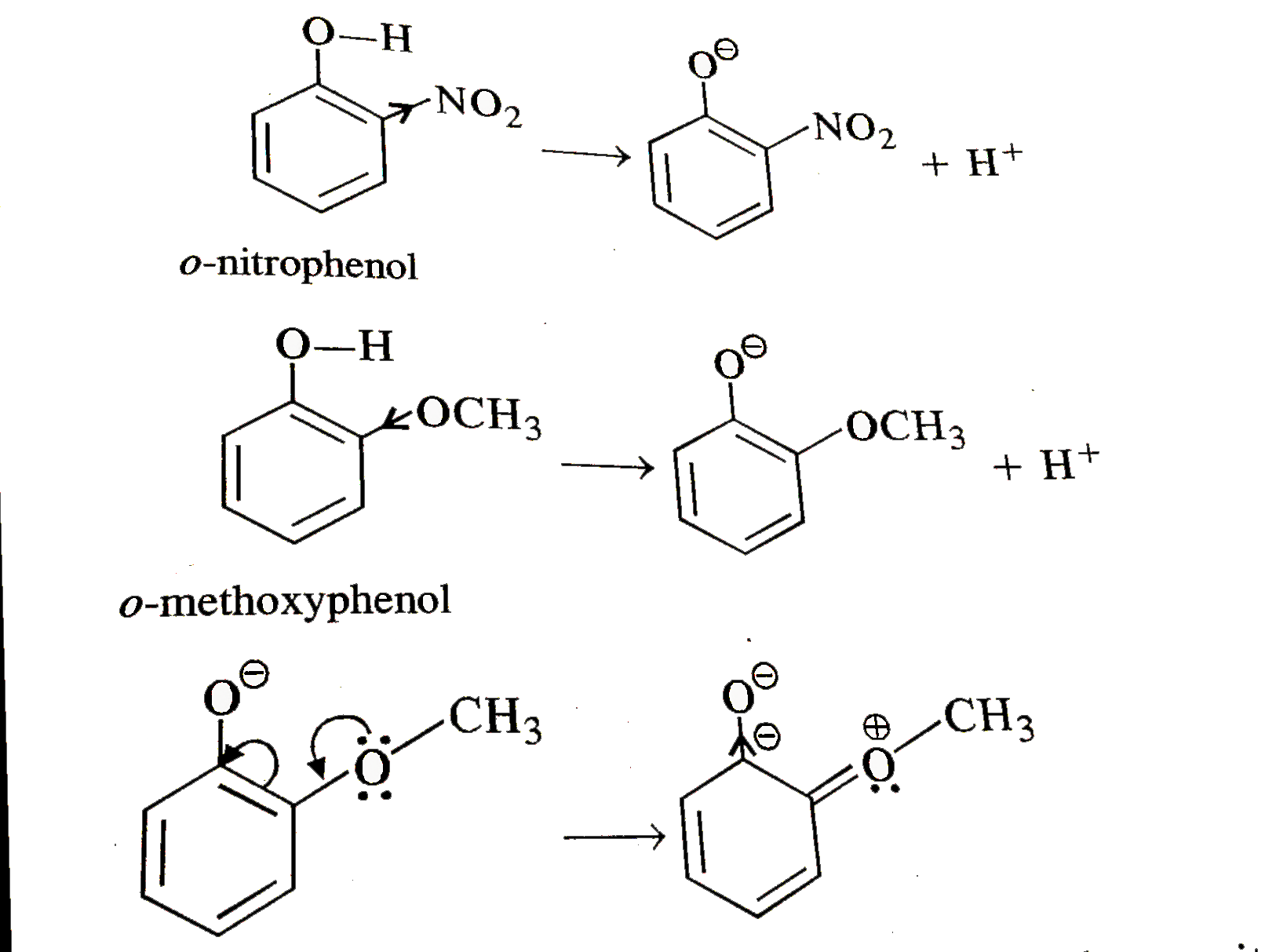
As a result, there is an increase in the electron density in the ring particularly at the ortho and para positions. the electrophilic substitution readily takes place at these positions when electrophile attacks.