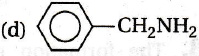
Correct Option
Explanation:
CH3 - [an electron releasing (+/) group] increases electron density at N-atom, hence basic nature is increased.

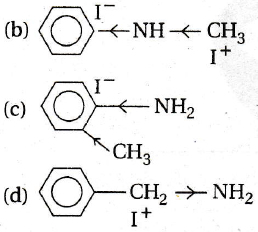
C6H5 decreases electron density at N-atom due to delocalisation of e - of NH2 with π e- of benzene Thus, basic nature is decreased. Hence, (d) is the strongest base.