In the graph plotted between vapour pressure (V.P.) and temperature (T),
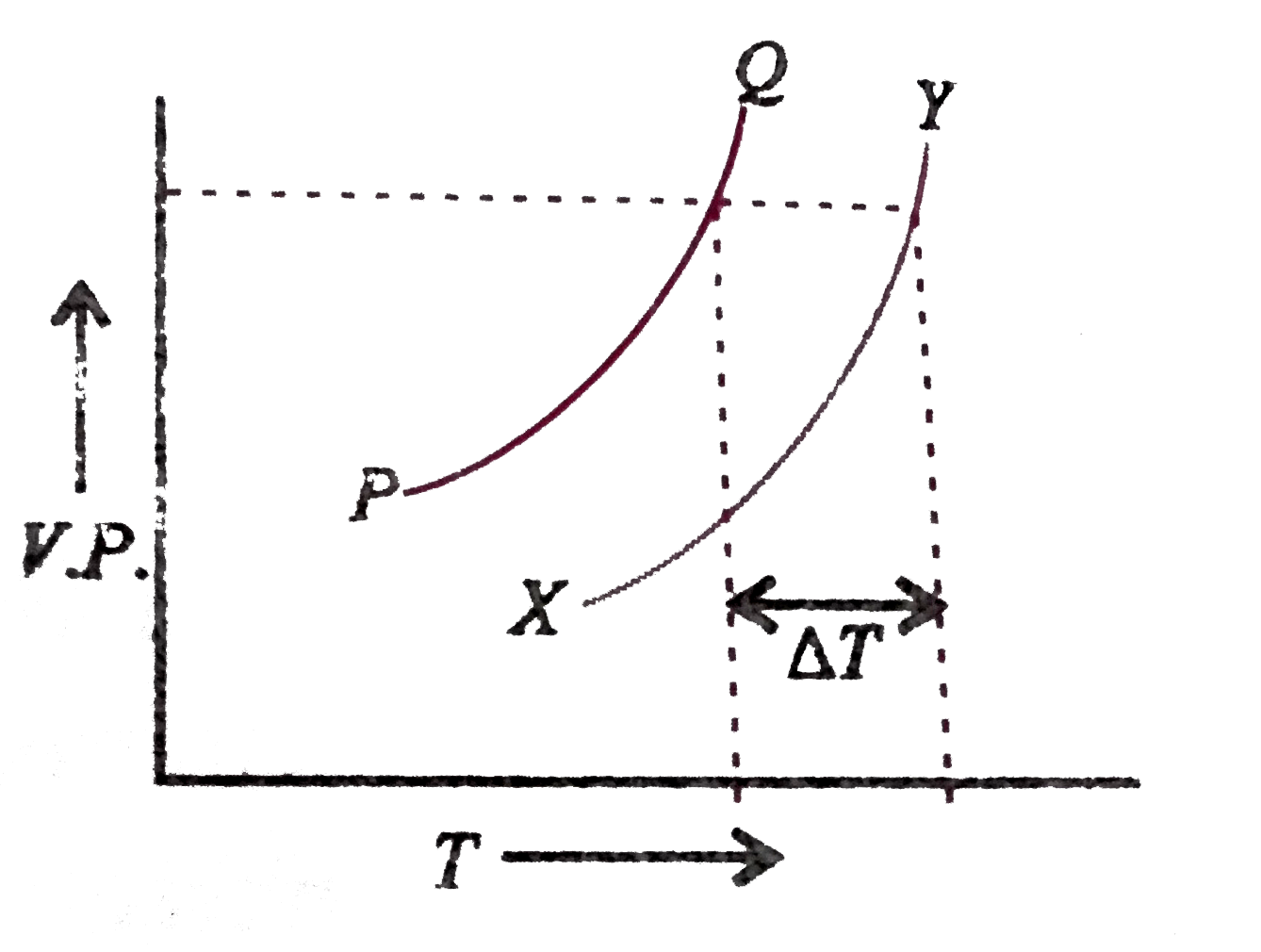
A. PQ is the curve for solvent , XY is the curve of solution and `DeltaT` is depression in freezing point
B. PQ is the curve for solution. XY is the curve for solvent and `DeltaT` is elevation is boiling point
C. PQ is the curve for solvent, XY is the curve for solution and `DeltaT` is molal elevation in boiling point
D. PQ is the curve for solvent, XY is the curve for solution and `DeltaT` is elevation in boiling point