Colloidal solutios generally contain excess amount of electrolytes and some other soluble impurities . It si necessary to reduce the concentration of soluble impuriteis to a requisite minimum . The impurities presence required in traces.
"The process used for reducing the amount of impurities to a requisite minimum is known as purification of colloidal solution.
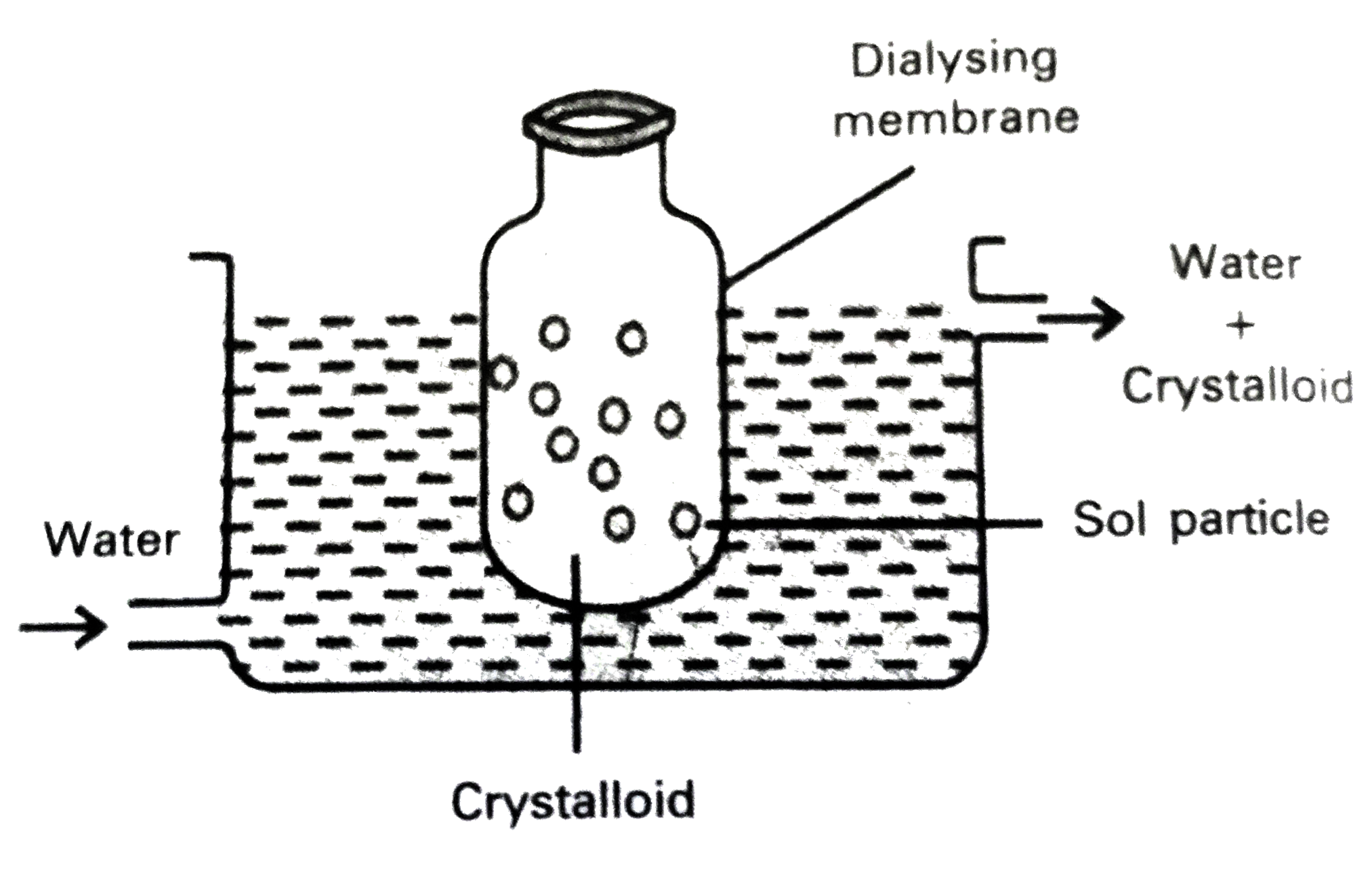
Purification of colloidal solution by Dialysis :
Dialysis : The process of removing a dissolved substance from a colloidal solution using a suitable membrane is called dialysis .
`to` In a true solution particles can pass through animal membrane (or) cellophone sheet (or) parchment paper but not colloidal particles.
`to` The apparatus used for the dialysis is called dialyser.
`to` A bag of suitable membrane containing the colloidal solution is suspended in a vessel containing a continuously flowing water.
`to` The molecules and ions diffuse through the membrane into the water and pure colloidal solution is left behind in the bag.