Correct Option (c) between 0.148 kJ and 0.028 kJ
Explanation:
Heat required to change the temperature of vessel by a small amount dT,

Work done required to maintain the temperature of sink to T2,
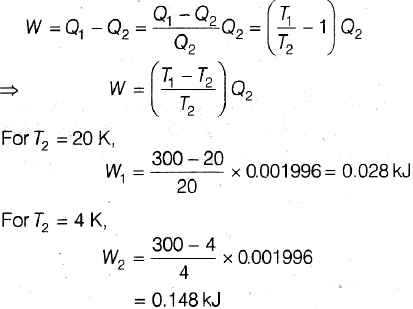
As temperature is changing from 20 K to 4 K, work done required will be more than W1, but less than W2.