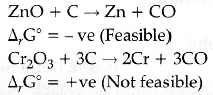(i) Cryolite performs two functions in the electrolysis of alumina
(a) It lowers the melting point of the mixture of about 1250K.
(b) It improves the electrical conductivity of the melt
(ii) The choice of a reducing agent in a particular case depends on thermodynamic factor. For a reaction to be feasible, the reaction of metal oxide with the reducing agent should have negative ΔG°.
Therefore, that reducing agent is suitable for which ΔG° for the reduction is negative. Thus