Kohlrausch law of independent migration of ions: The law states that limiting molar conductivity of an electrolyte can be represented by the sum of the individual contributions of the anion and cation of the electrolyte.
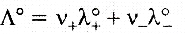
On dilution, the conductivity (k) of the electrolyte decreases as the number of ions per unit volume of solution decreases.