The reaction between (CH3) C-O-CH, and HI follows SN1 mechanism. For an SN1 reaction, the formation of product is decided by the stability of the cabocation formed in the slowest step. As tert- tertbutyl carbonium ion 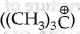 formed after the cleavage of C-O bond in the slowest step is more stable than methyl carbonium ion therefore (CH3)3 C-I and CH3OH are formed as main products.
formed after the cleavage of C-O bond in the slowest step is more stable than methyl carbonium ion therefore (CH3)3 C-I and CH3OH are formed as main products.