Given :
Atomic mass of Fe = 56,
S = 32, and
O = 16
Mass of iron, sulphur, oxygen and water = 0.2014 g, 0.1153 g, 0.2301 g and 0.4532 respectively.
To find :
The empirical formula of the compound
Calculation :
Since the mass of crystal is 1 g, the % iron, sulphur, oxygen and water = 20.14%, 11.53%, 23.01% and 4.32 % respectively.
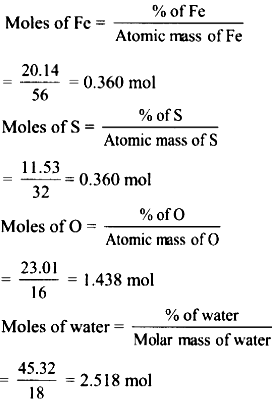
Hence,
The ratio of number of moles of Fe:S:O water is \(\frac{0.360}{0.360}\) = 1, \(\frac{0.360}{0.360}\) = 1, \(\frac{1.438}{0.360}\) = 4, \(\frac{2.518}{0.360}\) = 7
Hence,
Empirical formula is FeSO4.7H2O.
∴ Empirical formula of the compound = FeSO4.7H2O.