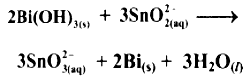Bi(OH)3(s) + SnO2-2(aq) ⟶ SnO2-3(aq) + Bi(s)
Step 1 :
Write unbalanced equation for the redox reaction. Assign oxidation number to all the atoms in reactants and products.
Divide the equation into two half equations.
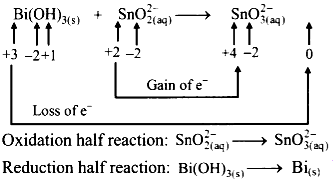
Step 2 :
Balance half equations for O atoms by adding H2O to the side with less O atoms.
Add 1H2O to left side of oxidation half equation and 3H2O to the right side of reduction half equation.
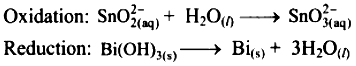
Step 3 :
Balance H+ atoms by adding H+ ions to the side with less H.
Hence,
Add 2H+ ions to the right side of oxidation half equation and 3H+ ions to the left side of reduction half equation.

Step 4 :
Now add 2 electrons to the right side of oxidation half equation and 3 electrons to the left side of reduction half equation to balance the charges.
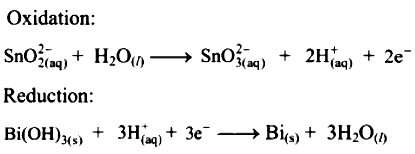
Step 5 :
Multiply oxidation half equation by 3 reduction half equation by 2 to equalize number of electrons in two half equations.
Then add two half equation.
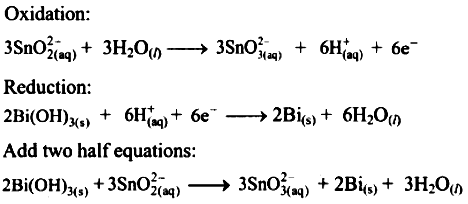
Reaction occurs in basic medium.
However,
H+ ions cancel out and the reaction is balanced.
Hence,
No need to add OH- ions.
The equation is balanced in terms of number of atoms and the charges.
Hence, balanced equation :