No
Because according to Bohr's model,
En = \(-\frac{13.6}{n^2}\) and electrons having different energies belong to different levels having different values of n.
So, their angular momenta will be different, as
L = mvr = \(\frac{nh}{2\pi}\)
OR
(i) The increase in the frequency of incident radiation has no effect on photoelectric current. This is because of incident photon of increased energy cannot eject more than one electron from the metal surface.

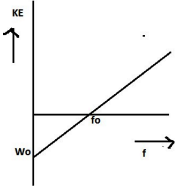
(ii) The kinetic energy of the photoelectron becomes more than the double of its original energy. As the work function of the metal is fixed, so incident photon of higher frequency and hence higher energy will impart more energy to the photoelectrons.