Correct option (b) More voltage is required to reduce H+ at Hg than at Pt
Explanation :
When sodium chloride is dissolved in water, it ionises as

Water also dissociates as
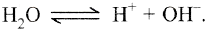
During passing of electric current through this solution using platinum electrode, Na+ and H+ ions move towards cathode. However, only H+ ions are discharged more readily than Na+ ion because of their low discharge potential (In the electromotive series hydrogen is lower than sodium). These H+ ions gain electrons and change into neutral atoms.
At cathode H+ + e → H, H + H → H2
Cl- and OH- ions move towards anode. Cl- ions lose electrons and change into neutral atom.
At anode, Cl- - e → Cl, Cl + Cl → Cl2
If mercury is used as cathode H+ ions are not discharged at mercury cathode because mercury has a high hydrogen over-voltage. Na+ ions are discharged at the cathode in preference to H+ ions, yielding sodium, which dissolves in mercury to form sodium amalgam. At cathode : Na+ + e = Na