Sodium carbonate (Na2CO3) is commercially prepared by Solvay process. Preparation of sodium carbonate by Solvay process involves two stages.
i. In the first stage of Solvay process, carbon dioxide gas is bubbled through a concentrated solution of NaCl which is saturated with NH3 . This results in the formation of ammonium bicarbonate. Crystals of sodium bicarbonate separate as a result of the following reactions.

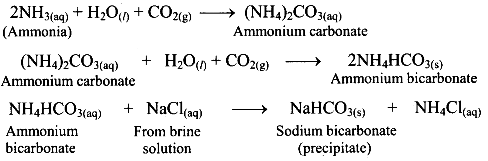
ii. Ammonium bicarbonate and sodium chloride undergoes double decomposition reaction to form sodium bicarbonate. As sodium bicarbonate has low solubility, it precipitates out in the form of crystals. iii. In the second stage, the separated crystals of sodium bicarbonate are heated to obtain sodium carbonate (Na2CO3).
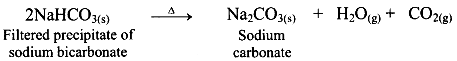
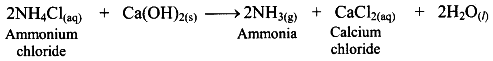
iv. NH4Cl obtained in this process is treated with slaked lime, Ca(OH)2 , to recover NH3 while CaCl2 is obtained as a byproduct.