i. The bond structure shows that there is no π bond. Therefore, no resonance and no resonance stabilization.
ii.
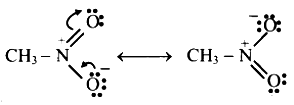
N = O double b5itd is attached to ‘O’ which carries lone pair of electrons in a p orbital. Therefore, resonance structures can be written as shown and species is resonance stabilized.
iii.

The Lewis structure shows two C = C double bonds alternating with a C – C single bond. Therefore, resonance structures can be written as shown and the species is resonance stabilized.