Rupali, a class 10 student has set up the apparatus as shown in the figures.
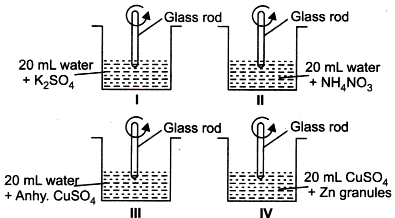
Which of the following observations is correct?
(A) Temperature of beakers l, ll and lll will be raised as dissolution of salts is an exothermic process.
(B) Temperature of beakers lll and lV will be raised while temperature of beakers I and ll will fall.
(C) Temperature will rise only in beaker lV as redox reactions are exothermic.
(D) None of these