Linus Pauling was proposed valence bond theory.
Formation of N2 molecule :
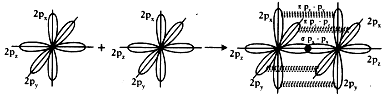
1. Electronic configuration of Nitrogen is 1s2 2s2 2px1 2py1 2pz1.
2. Suppose that px orbital of one Nitrogen atom overlaps the px orbital of other ‘N’ atom giving σ px – px bond along the inter nuclear axis.
3. The py and pz orbitals of one ‘N’ atom overlaps with the py and pz orbital of other ‘N’ atom laterally giving π py – py and π pz – pz bonds.
4. Therefore, N2 molecule has a triple bond between two Nitrogen atoms.
Formation of O2 molecule :
1. Electronic configuration of ‘O’ is 1s2 2s2 2px1 2py1 2pz1.
2. If the Py orbital of one ‘O’ atom overlaps the py orbital of other ‘O’ atom along internuclear axis, a σ py – py bond is formed.
3. pz orbital of oxygen atom overlaps laterally, perpendicular to inter nuclear axis giving a π py – pz bond.
4. So O2 molecule has a double bond between the two oxygen atoms.
