Sulphuric acid (H2SO4 ) is a dibasic acid as explained below:
(1) It ionises in aqueous solution to produce two hydrogen ions per molecule of the acid.
OR
It contains two replace all hydrogen ions per molecule of the acid.
H2SO4 (aq) ⇋ 2H+(aq) + SO42-(aq)
(2) It ionises in two steps in aqueous solution as shown below:
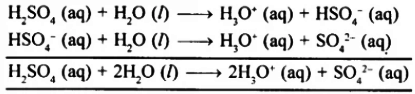
(3) It forms two types of salt, e., normal salt and acid salt as shown below:
