Let us consider a first order reaction,
A → product
Suppose at the beginning of the reaction, that is, when t=0, the concentration of A is amol L-1 and when t = t, the concentration of A is (a-x)mol L-1.
For a first order reaction, the rate of reaction,
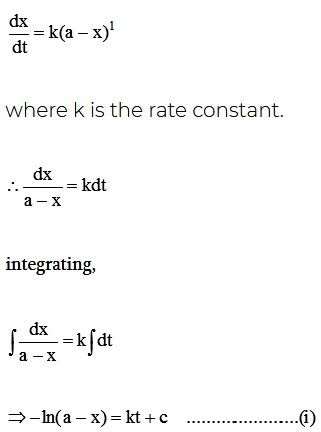
Here, c is the constant of integration.
At, t = 0, x = 0 ; then from equation (i) we have,
c = -In a
putting the value of c in equation (i), we get,
ln a – ln(a-x) = kt
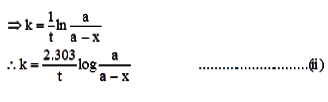
Equation (ii) is the required expression.
Unit of k:
From equation (ii) we found that the unit of k depends on the unit of t . The unit of k may be s-1, min-1, h-1, day-1 and so on.
