1. The equation for the laboratory preparation of hydrogen chloride gas :
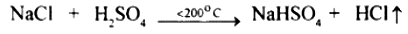
Although it is a reversible reaction, it goes to completion as hydrogen chloride continuously escapes as a gas.
The reaction can occur up to the stage of the formation of sodium sulphate on heating above 200°C.
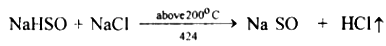
2. The drying agent used in the laboratory preparation of hydrochloric acid is conc.sulphuric acid. The other drying agents such as phosphorus pentoxide (P2O5 ) and quick lime (CaO) cannot be used because they react with hydrogen chloride.

3. A safety precaution which should be taken during the preparation of hydrochloric acid : Always wear chemical splash goggles, chemical-resistant gloves and a chemical resistant apron in the laboratory during the preparation of hydrochloric acid.