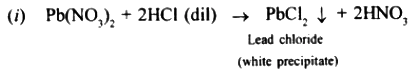
When dilute hydrochloric acid is added to lead nitrate white precipitates of lead chloride is formed.
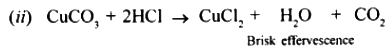
When dilute hydrochloric acid is added to copper carbonate brisk effervescence due to the liberation of carbon dioxide is observed.

When dilute hydrochloric acid is added to sodium thiosulphate pale yellow residue (due to formation of sulphur) is formed and a gas with choking odour is formed.