Aim :
To separate ammonium chloride from the mixture of ammonium chloride and common salt.
Materials required :
China dish, funnel, cotton, ammonium chloride, common salt and stove.
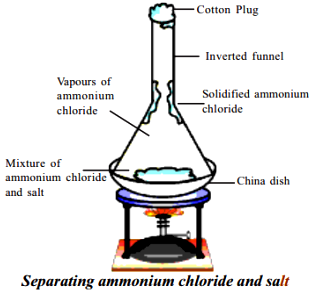
Procedure:
- Take one table spoon of ammonium chloride and one table spoon of common salt and mix them.
- Take the mixture in a China dish.
- Take a glass funnel.
- Plug the mouth of the funnel with cotton.
- Invert the funnel over the dish.
- Heat the dish on the stove and observe the walls of the funnel.
Observations :
Initially we find vapours of ammonium chloride and then solidified ammonium chloride on the walls of the funnel.