When equal volumes of the acid and water are mixed at room temperature, the temperature may reach up to 120°C. Therefore, dilution of the acid should be done by adding small quantity of acid into water.
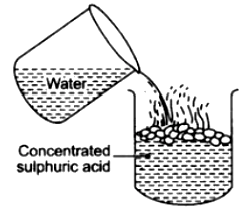
If water is added to concentrated sulphuric acid, the molecules of the acid try to grasp the molecules of water resulting in molecular tension, liberating heat and due to sudden rise in temperature, the acid starts splashing. If a drop of concentrated acid is added to water, the molecules of acid go in different directions to pick up water which is available in plenty. Although the same amount of heat is formed but since the molecules are spread out, no splashing occurs.