(i) Dilute sulphuric acid reacts with metals above hydrogen in the activity series, for example, magnesium to liberate hydrogen gas and magnesium sulphate.
Mg + H2SO4 ⟶ MgSO4 + H2 ↑
(ii) When sulphur is heated with concentrated sulphuric acid, it is oxidised to sulphur dioxide. S + 2H2SO4 ⟶ 3SO2 (Sulphur dioxide) + 2H2O
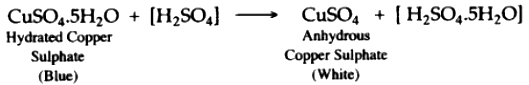
(iii) Add few drops of concentrated sulphuric acid to blue coloured crystals of copper (II) sulphate. After sometime, white anhydrous copper (II) sulphate is left, due to loss of water of crystallization.
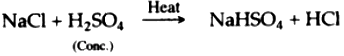
(iv) Concentrated sulphuric acid, when heated with sodium chloride, produces volatile hydrochloric acid.