Borax on heating loses its water molecules firstly and on further heating it melts into a clear liquid which solidifies to a transparent glass like bead which consists of sodium metaborate (NaBO2) and boric anhydride.
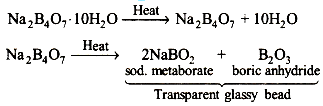
From this glass bead, B2O3 is heated with K or Mg in absence of air, therefore boron is obtained by reduction,
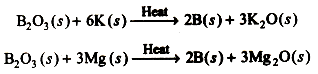
The product thus obtained b boiled with HCI and filtered when K2O or MgO dissolves leaving behind elemental boron. the powder (thus boron) is obtained throughly washed with water to from it from HCl and then, it is dried. Following reaction is possible between B2H6 and HCI
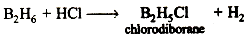
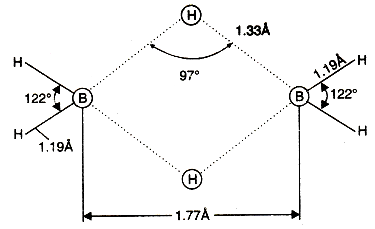
The structure of compound (B2H6) : In it two electrons of a B-H bond are involved in formation of three centre bond (Banana bond), these two bonds are represented as dotted lines in following structure, in which one lies above and second lies below to the main plane.