Aldehydes and ketones ar specially susecptible to nucleophilic addition because carbonyl group

is polar (due to electronegativity difference between carbon and oxygen)

Positive charge on carbon makes it reactive towards the nucleophili. This addition is catalysed by acid.
Reactivity of carbonyl compound towards nucleophilic addition increases with increases in the electron deficiency at carbonyl carbon. Thus, (-I.E.)groups increase while (+I.E.) groups decrease the reactivity of carbonyl compound.
Which among teh following carbonyl compounds is most pola?
A.
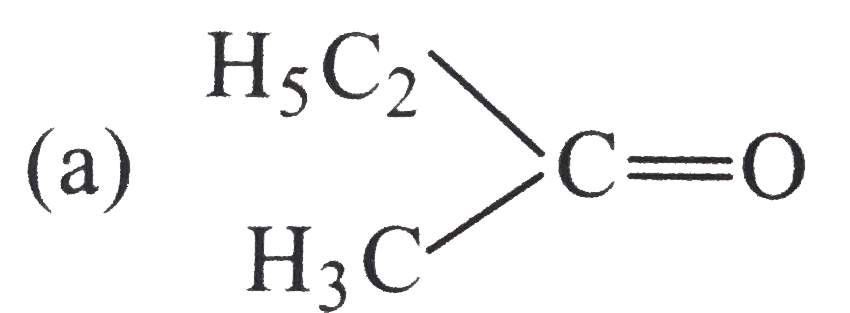
B.
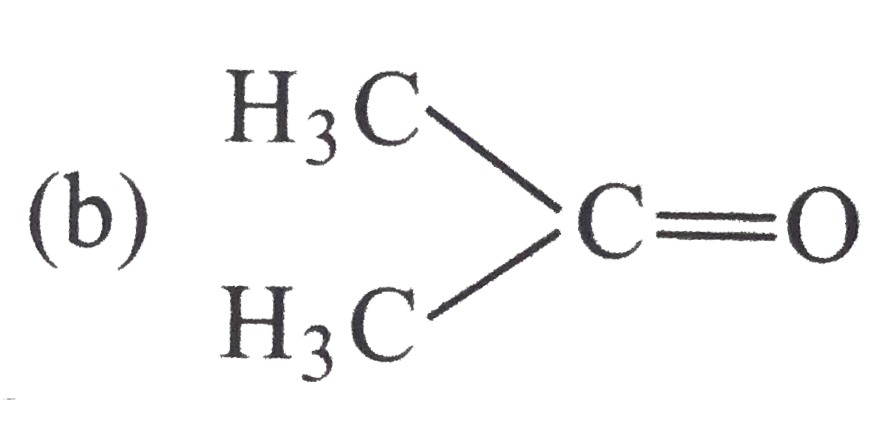
C.

D.
