
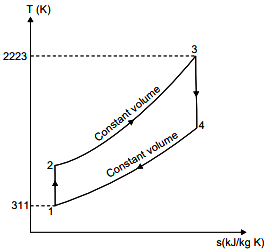
Initial temperature, T1 = 38 + 273 = 311 K
Maximum temperature, T3 = 1950 + 273 = 2223 K.
(i) Compression ratio, r :
For adiabatic compression 1-2,
p1V1γ = p2V2γ

Hence compression ratio = 6.9.
(ii) Thermal efficiency :
Thermal efficiency,

(iii) Work done :
Again, for adiabatic compression 1-2

For adiabatic expansion process 3-4

Heat supplied per kg of air
= cv(T3 – T2) = 0.717(2223 – 671.7)
= 1112.3 kJ/kg or air

Heat rejected per kg of air
= cv(T4 – T1) = 0.717(1029 – 311)
= 514.8 kJ/kg of air
∴ Work done per kg of air = Heat supplied – heat rejected
= 1112.3 – 514.8
= 597.5 kJ or 597500 N-m.