Correct option (b)
Explanation:
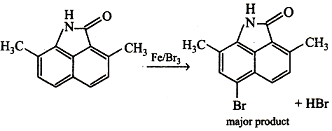
It is electrophilic substitution, so electrophile must be attacked on o/p-position due to higher electron density on these position. In this ring the attached -NH- group will have high electron density due to resonance and ortho position is blocked, so electrophile is attacked on para position.