When a bond is formed by sharing of electrons by the two atoms the bond formed between them is called covalent bond. These mutually shared electrons become the common property of both atoms and also counted towards their stable configuration. Each pair of shared electrons is indicated by a line (-----).
For example during formation of H2 molecule, the two hydrogen atoms mutually share their electron. It is represented as.
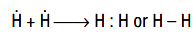
Once the covalent bond has been formed, the two bonding electrons are attracted by the two nuclei instead of one, so the bonded state becomes more stable than the non-bonded state. The number of covalent bonds formed by an atom is termed as its covalency. The covalency of an atom will be equal to the number of electrons, the atom needs to become iso-electronic with a noble gas. For example, in the formation of water molecule (H2O), covalency of oxygen is two and that of hydrogen is one as shown below.
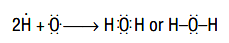
The covalent bond formed by the mutual sharing of one pair of electrons between two atoms is called a single bond. It the electron pairs shared between two atoms are two or three then the bond is said to be double or triple, respectively.