a)
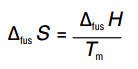
∆fus H = enthalpy of fusion per mole
Tm = melting temperature in Kelvin
b)

∆fusH = enthalpy of vapourization per mole
Tb = boiling temperature in kelvin
c)

∆sub H = enthalpy of sublimation at the temperature T.
• For all spontaneous processes, the total entropy change must be positive
∆Stotal = ∆Ssystem +∆Ssurr > O
∆Stotal = +ve ⇒ Process is spontaneous
Stotal = -ve ⇒ Direct process is non-spontaneous; the reverse process is spontaneous.
• When a system is in equilibrium, the entropy is maximum and the change in entropy, ∆S= O.
• ∆U does not discriminate between reversible and irreversible process whereas ∆Sdoes.
∆U = O (for both reversible & irreversible expansion for an ideal gas; under isothermal conditions)
∆Stotal > O (for irreversible process) and ∆Stotal = O (for reversible process)