The expression for pressure, P; exerted by a gas is
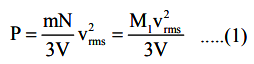
Let E = 1/2 M1\(V^2_{rms}\) The total kinetic energy of atoms or molecules of the gas. In terms of energy, E, Eqn. (1) is rewritten as

According to the ideal gas equation

where M is molar mass of gas. From Eqns. (2) and (3) we have
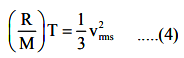
Let NAV be the Avogadro’s number. It is the number of atoms or molecules in one–mole of gas. Obviously M = mNAV. Using this we rewrite Eqn (4) as

R/NAV = K = Boltzmann’s constant. Therefore
1/2 mv\(^2_{rms}\) = Kinetic energy of one atom or molecules of gas = 3/2 kT ....(6)
From Eqn (6) we conclude that temperature of gas (T) is a measure of the translational kinetic energy of the atoms / molecules of gas. This is the kinetic interpretation of temperature. From Eqn. (1);

At T = 0K; vrms = 0. In other words absolute zero of temperature is that temperature at which there is no motion of atoms / molecules of gas.