(i) Hydrogen chloride is produced.
KCl + H2SO4 → KHSO4 + HCl
(ii) Sulphuric acid, being a strong acid displaces relatively weaker nitric acid from its salt.
KNO3 + H2SO4 → KHSO4 + HNO3
(iii) It decomposes sodium carbonate to carbon dioxide and water.
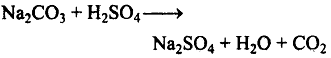
(iv) It oxidises Br– to Br2.
