A carboxyl group is made up of a carboxyl group joined to a hydroxyl group.

In the carboxyl group, the carbon atom is attached to two carbon atoms: one by a double bond and the other by a single bond which in turn linked to a hydrogen atom by a single bond. The remaining free valency of the carbon atom of the carboxyl is satisfied by a H atom or an alkyl group. Thus, the structure of carboxylic acids.
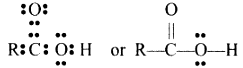
where ‘R’ is ‘H’ or alkyl group.
The carbon atom and the two oxygen atoms are sp2 hybridised. The two sp2 hybridesed orbitals of the carboxyl carbon overlap with one sp2 hybridised orbital of each oxygen atom while the third sp2 hybridised orbital of carbon overlaps with either a ‘s’ orbital of H – atom or a sp3 hybridised orbital of ‘C’ atom of the alkyl group to form three a bonds. Each of the two oxygen atoms are left with one unhybridised orbital which is perpendicular to the a bonding skeleton.

All the three ‘p’ orbitals being parallel, overlap to form n bond which is partly delocalised between carbon and oxygen atoms on one side and carbon and oxygen of the ‘OH’group on the other side.

In other words, RCOOH may be represented as a resonance hybrid of the following two canonical structures.

As a result of resonance (i) the C—O single bond length in carboxylic acids is shorter than the normal C—O single bond in alcohols and ethers and (ii) C=O bond length in carboxylic acids is slightly larger than the normal C=O bond length in aldehydes and ketones.