Higher the Ka value, the stronger is the acid. Thus, p-nitro benzoic acid is a stronger acid than benzoic acid. This is due to
1. Because of — I and -M effect of NO2 group the electron density in O—H bond decreases. As a result, the —O—H bond becomes weak and hence, p – nitrobenzoic acid easily loses a proton than benzoic acid.
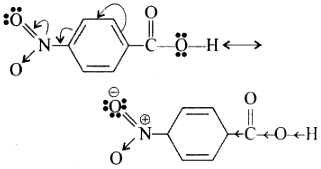
2. Due to -1 and -M effect of nitrogroup dispersal of negative charge occurs and hencep -nitro benzoate ion becomes more stable than benzoate ion.
