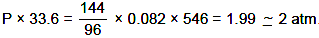Correct option: (d) 2 atm
Explanation:
Molecular wt. of monomer = 48 gm
so molecular wt. of dimer will be 98 gm
In experiment, Mass of compound = 96 gm
Volume of vessel = 33.6 L
Temperature = 273° C → 273 + 273 = 546 K
If compound exist as a dimer then mass extent of mass by 50% of wt.
so 50% of 96 = 48 gm
Now weight = 96 + 48 = 144 gm
Pv = nRT