Identify the correct order of reactivity for the following pairs towards the respective mechanism
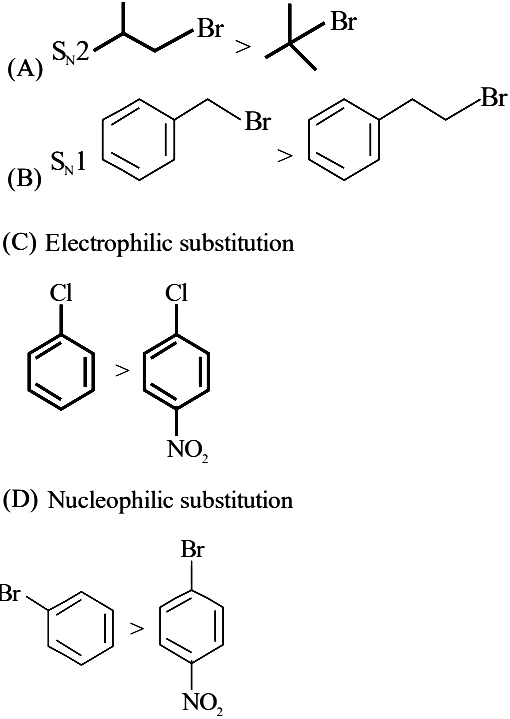
Choose the correct answer from the options given below :
(1) (A), (B) and (D) only
(2) (A), (B), (C) and (D)
(3) (A), (C) and (D) only
(4) (B), (C) and (D) only