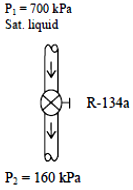
Refrigerant-134a is throttled by a valve. The temperature drop of the refrigerant and specific volume after expansion are to be determined.
Assumptions:
- This is a steady-flow process since there is no change with time.
- Kinetic and potential energy changes are negligible.
- Heat transfer to or from the fluid is negligible.
- There are no work interactions involved.
Properties: The inlet enthalpy of R-134a is, from the refrigerant tables (Tables A-11 through 13).
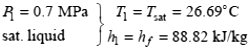
Analysis: There is only one inlet and one exit, and thus \(\overset •m_1 = \overset •m_2 = \overset •m\). We take the throttling valve as the system, which is a control volume since mass crosses the boundary. The energy balance for this steady-flow system can be expressed in the rate form as
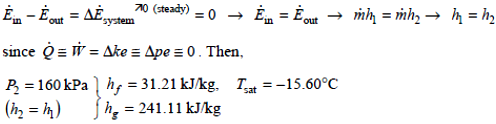
Obviously hf < h2 < hg, thus the refrigerant exists as a saturated mixture at the exit state and thus T2 = Tsat = -15.60°C. Then the temperature drop becomes
\(\Delta\)T = T2 - T1
= -15.60 - 26.69
= - 42.3°C
The quality at this state is determined from
\(x_2 = \frac{h_2 - h_f}{h_{fg}}\)
\(= \frac{88.82 - 31.21}{209.90}\)
= 0.2745
Thus,
v2 = vf + x2vfg
= 0.0007437 + 0.2745 x (0.12348 - 0.0007437)
= 0.0344 m3/kg