(a) The zero order wave function of the two electrons has the forms

being the normalized symmetric (+) and antisymmetric (-) wave functions, x0 and x1 denote the singlet and triplet spin states respectively. Denoting \(u_{1s}(1)v_{2s}(2) \) by \(|1,2\rangle\) and \(u_{1s}(2)v_{2s}(1)\) by \(|2,1\rangle\), we can write the above as

Because the perturbation Hamiltonian is independent of spin, we need not consider x. Thus
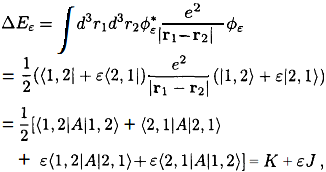
where \(A =e^2|r_1 - r_2|, K = \langle 1,2|A|1,2\rangle = \langle 2,1|A|2,1\rangle\)is the direct integral, \(J = \langle 1,2|A|2,1\rangle = \langle 2, 1|A| 1,2\rangle \) is the exchange integral.
(b) The singlet state x0 is antisymmetric for interchange of spins. The triplet state x1 is symmetric for interchange of spins. Similarly, \(\phi_+\) is symmetric for the interchange of r1 and r2, and \(\phi_-\) is antisymmetric for the interchange. Hence the total wave function is always antisymmetric for interchange of the electrons.
(c) The initial state of the system is

which shows that at this time the 1s electron has spin down and the 2s electron has spin up i.e., the spins are reversed. As -1 = ei(2n+1)π, n = 0,1, 2..., this happens at times
