(c) A ring structure
Glucose reacts with methanol, in presence of dry HCl, to form alpha and beta methyl glycosides.
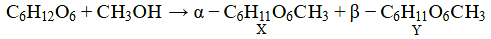
Formation of alpha and beta methyl glycosides is due to the ring structure of glucose, as the methyl alcohol can attack the anomeric carbon in 2 ways, leading to these two configurations.