Swarts Reaction
Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or alkyl bromides. This is done by heating of the alkyl chloride/bromide in the presence of the fluoride of some heavy metals (silver fluoride or mercurous fluoride for example). The reaction will proceed if sodium fluoride or potassium fluoride is used, but the resulting yield will be significantly lower. This process was first reported by Frederic Jean Edmond Swarts in 1892.
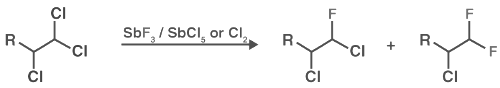
In Swarts fluorination, chlorine is most commonly replaced by fluorine in organic compounds with the help of antimony trifluoride in the presence of antimony salts where its oxidation state is +5. A few examples of this type of reaction can be found below:
The Swarts reaction mechanism is quite simple – the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. The displaced chlorine or bromine atoms now bond with the metal. The mixture of antimony trifluoride and chlorine is referred to as Swarts reagent. Swarts rule states that post fluorination, the fluoride formed will always have a lower boiling point than the corresponding chloride.
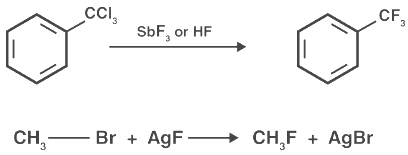
Chlorinated hydrocarbons can also be reacted with metallic fluorides to give hydrocarbons with chlorine and fluorine attached. For example:

Thus, Swarts fluorination can be used for the complete replacement of chlorine or bromine with fluorine in alkyl chlorides/bromides. A variant of the reaction is quite important in the production of Freons. In this variation, the fluorination is executed using anhydrous hydrogen fluoride in the presence of antimony salts (of catalytic quantities) where antimony displays oxidation levels of +3 and +5.