Effects of Hydrogen Bonding-
1. Boiling point: The boiling point of a liquid is the temperature at which kinetic energy of the molecule is sufficient to overcome the intermolecular attractive forces. Thus it seems to be reasonable that heavier the molecule and stronger the intermolecular forces higher will be the boiling point of the compound. Thus, if the intermolecular forces are equal, the melting and boiling points of similar compounds should increase with the increase in molecular weight of the compound. This explains why the melting and boiling points generally increase with increase in number of carbon atoms in most of the homologous series.
On the other hand, if the two similar compounds under study are having different intermolecular forces, their melting and boiling points differ very much. It is due to the fact that molecules of the compound having greater attractive intermolecular force have a greater tendency of association with the result the effective molecular weight and subsequently melting and boiling points of the compound increase. Best familiar examples of this type of compounds are water, alcohols, hydrogen fluoride, amines and amides.
2. Water solubility: A substance is said to be soluble in water if it is capable of forming hydrogen bonding with water molecules. On the other hand, compounds in which hydrogen bonding with water is not possible (or restricted due to intramolecular hydrogen bonding) would be insoluble or less soluble in water. Thus organic compounds like alkenes, alkanes and ethers which lack the formation of hydrogen bonds with water are insoluble in water while alcohols and acids which are capable of forming hydrogen bonds with water are readily soluble in water.
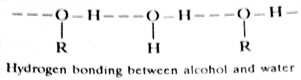
However, it is important to note that when the alkyl group (R-) in alcohols has four or more carbon atoms, the alkyl group predominates and hydrogen bond formation is restricted with the result the solubility of such alcohols in water decreases. Alcohols containing more than seven carbon atoms are insoluble in water, while methyl, ethyl and propyl alcohols are fairly soluble in water.
When a compound has a large ratio of - OH groups to hydrocarbon group, it will be very much soluble in water. Thus sugars, certain starches and polyvinyl alcohol are quite soluble in water.
Thus we can explain the solubility of alcohols, phenols, primary and secondary amines in water since these compounds easily form hydrogen bonds with water molecules. The success of non-ionic detergents is due to the powerful solubilizing effect of hydroxyl groups. Since the water soluble portion of these detergents has neutral hydroxy, ether or amino groups, they are easily dissolved in water as a result of hydrogen bonding.
The insolubility of tertiary amines in water is probably due to a steric effect rather than lack of N---H-O bonding because tertiary amines with the alkyl groups tied back by ring formation, such as pyridine, are soluble in water.
It is important is note that while the intermolecular hydrogen bonding increases solubility of the compound in water, the intramolecular hydrogen bonding decreases. This is due to the fact that the formation of internal hydrogen bond prevents hydrogen bonding between the compound and water which thus reduces solubility of the compound in water.
One practical application of the contrasting effects of chelation and association upon boiling point and water solubility is made in the separation of a mixture of ortho- and para-hydroxy- carbonyl or - nitro compounds by steam distillation. In case of ortho isomers, association of the hydroxyl group with water molecules is prevented due to chelation and thus these isomers are only sparingly soluble in water and possess lower boiling points and are thus much volatile that the para isomers with the result the ortho isomers can be steam distilled so much rapidly than the para isomers that a practical separation of the two isomers can be achieved.
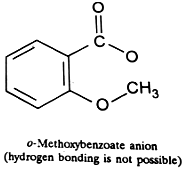
3. Strength of acids: The dissociation of an acid and hence its acidity is sufficiently increased, if its anion is stabilized by chelation. This can be proved by the ionization constants of the three isomeric hydroxy- and methoxy-benzoic acids in water.
|
ortho |
meta |
para |
| OH.C6H4.COOH |
105 x 105 |
8.3 x 105 |
2.9 x 105 |
| OCH3.C6H4.COOH |
8.1 x 105 |
8.2 x 105 |
3.4 x 105 |
The abnormally high dissociation constant of the o-hydroxybenzoic acid in the above table is due to the fact its anion is stabilized by chelation. Its dissociation is 17 times more than that of benzoic acid. The fact that hydrogen bonding increases the acidity of the o-isomer is proved by the very low
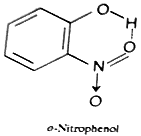
[Due to chelation, -OH group is not available to form hydrogen bond with water hence it is sparingly soluble in water. Further, association of the molecules due to intermolecular hydrogen bonding is also not possible hence it has high to p-Nitrophenol it has lower boiling point.]
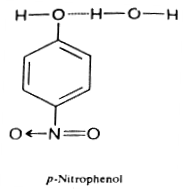
[-OH group available to form hydrogen bond with water, hence it is completely soluble in water. Chelation is not possible, hence association of the molecules is possible due to intermolecular hydrogen bonding. Hence it has high boiling point.]
dissociation constant of the o-methoxybenzoic acid where hydrogen is not available for hydrogen bonding. Furthermore, the dissociation of 2, 6-dihydroxybenzoic acid is 800 times as large as that of benzoic acid which is again due to the greater stablization of its anion by hydrogen bonding.
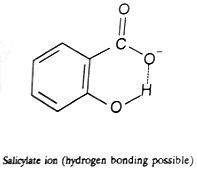
Similarly, we can explain very strong acidic character of maleic acid than fumaric acid. If one proton is removed from each of the acids, the corresponding ions are formed. But the maleate ion can be stabilised by chelation because hydrogen and oxygen responsible for forming hydrogen bond are very near to each other.
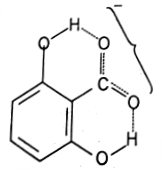
2,6-Dihydroxybenzoate anion (hydrogen bonding possible)
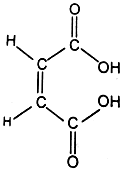
Maleic acid (chelation not possible)
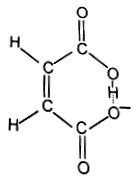
Maleate ion (chelation possible)
On the other hand, fumarate ion can not stabilise by chelation because hydrogen and oxygen are on the opposite sides to each other, hence the formation of fumarate ion does not take place. This explains why maleic acid is a stronger acid than its isomer, fumaric acid.

Fumaric acid (chelation not possible)

Fumarate ion (chelation not possible)

In case, the dissociating hydrogen atom is involved in hydrogen bond, enough energy will be required for its removal and thus the dissociation constant will be abnormally low. This explains why the o-halogeno phenols are weaker acids than the m- or p-isomers.
Similarly, we can explain why HF is a less stronger acid than any other halogen acid. Since HF is capable of forming strong hydrogen bonds, its proton is dissociated with a great difficulty as compared to other halogen acids. Thus acidity among halogeno acids follows the following order:
HF < HCl < HBr < HI
4. Basic strength of amines: Moore and Winmill observed that the compounds like NH3,MeNH2,Mc2NH and Me3N are weak bases, while the quaternary compound Me4N+OH- is a very strong base. They explained this marked change from Me3N to Me4NOH- in the following way.
When dissolved in water, the first four bases combine with water to yield species I which owing to the formation of hydrogen bond can not be regarded as fully ionic, II.

On the other hand, the tetramethyl ammonium hydroxide, lacking a hydrogen atom on the nitrogen, cannot exist in a form analogous to I. It exists only in the completely ionized form III with the result the compound is a strong base, quite comparable in strength to potassium hydroxide.