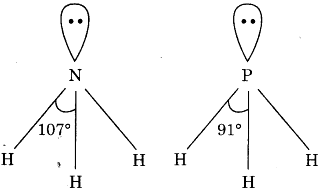
NH3 and PH, both are hydrides of elements of group 15. The electronegativity of nitrogen is more than phosphorus; consequently, shared electron pair in N-H bond is more towards nitrogen whereas in P-H bond this shared pair of electron is less towards phosphorus. Due to more electronegativity of nitrogen, electron density around nitrogen atom increases and repulsion increases. Hence, in NH3, H - N - H bond angle is also 107° while in H-P-H, it is 91°.