Correct option is : (1) 0
\(E _{Fe^{3+}/Fe^{2+}} ^{o} = 0.77 V\)
\(E _{Co^{3+}/Co^{2+}} ^{o} = 1.97 V \)
\(E _{Cr^{3+}/Cr^{2+}} ^{o} = -0.41 V\)
\(E _{Mn^{3+}/Mn^{2+}} ^{o} = 1.57 V\)
Cobalt having maximum value of \(E _{Co^{3+}/Co^{2+}} ^{o} \) i.e. 1.97 V
Complex compound should be [Co(CN)6]3–
Co3+ ⇒ [Ar]3d6 4s0
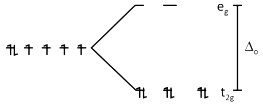
\(\Delta o\) > P as CN– is a SFL with Co3+ ion
\(t _{2g}^{6} e_{g}^{0}\)
Number of electrons in eg set of orbitals = 0