σ- bond can be formed by any of the following types of combinations of atoms orbitals.
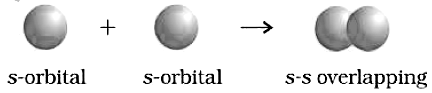
(a) S – S – overlapping : In this case, there is a lover lap of two half – filled S – orbitals along the inter nuclear axis.
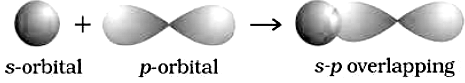
(b) S- P overlapping : This type of over lapping occurs between half – filled s-orbitals of one atom and half-filled p-orbitals of another atom.
(c) P – P overlapping : This type of overlap takes place between half-filled p-orbitals of the two approaching atoms.
