Vapour density of mixture = 38.3
Molecular mass of mixture = 2 38.3
Mass of mixture = 100 g
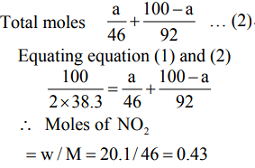
Number of moles present = 100/2 x 38.3...(1)
Suppose mass of NO a gram
Mass of N2O4 (100 – a)gram
Moles of NO2 = w / M = a / 46
Moles of N2O4 = w / M (100 - a)/92