An ideal gas is made to undergo a cycle depicted by the PV diagram alongside. The curved line from A to B is an adiabat,
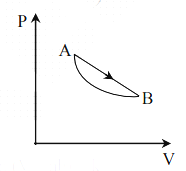
Then-
(A) The efficiency of this cycle is given by unity as no heat is released during the cycle
(B) Heat is absorbed in the upper part of the straight line path and released in the lower part
(C) If T1 and T2 are the maximum and minimum temperatures reached during the cycle, then the efficiency is given by 1 – T1 / T2
(D) The cycle can only be carried out in the reverse of the direction shown in figure