In 1913, Niels Bohr Bohr, combined classical and early quantum concepts and gave his theory in the form of three postulates. These are :
(i) Bohr’s first postulate was that an electron in an atom could revolve in certain stable orbits without the emission of radiant energy, contrary to the predictions of electromagnetic theory. According to this postulate, each atom has certain definite stable states in which it can exist, and each possible state has definite total energy. These are called the stationary states of the atom.
(ii) Bohr’s second postulate defines these stable orbits. This postulate states that the electron revolves around the nucleus only in those orbits for which the angular momentum is some integral multiple of h/2π where h is the Planck’s constant (= 6.6 × 10–34 J s). Thus the angular momentum (L) of the orbiting electron is quantised. That is
L = nh/2π .......(1)
(iii) Bohr’s third postulate incorporated into atomic theory the early quantum concepts that had been developed by Planck and Einstein. It states that an electron might make a transition from one of its specified non-radiating orbits to another of lower energy. When it does so, a photon is emitted having energy equal to the energy difference between the initial and final states. The frequency of the emitted photon is then given by h
ν = Ei – Ef ----------- (2)
where Ei and Ef are the energies of the initial and final states and Ei > Ef. For a hydrogen atom, Eq.(1) gives the expression to determine the energies of different energy states. But then this equation requires the radius r of the electron orbit. To calculate r, Bohr’s second postulate about the angular momentum of the electron the quantisation condition – is used. The angular momentum L is given by
L = mvr
Bohr’s second postulate of quantisation Eq. (1) says that the allowed values of angular momentum are integral multiples of h/2π.
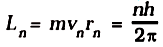
where n is an integer, rn is the radius of nth possible orbit and vn is the speed of moving electron in the nth orbit. The allowed orbits are numbered 1, 2, 3 ..., according to the values of n, which is called the principal quantum number of the orbit.
From Eq. (3), the relation between vn and rn is
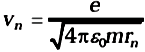
Combining it with Eq. (3), we get the following expressions for vn and rn,
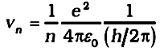
and
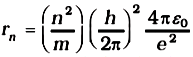
Eq. (4) depicts that the orbital speed in the nth orbit falls by a factor of n. Using Eq. (5), the size of the innermost orbit (n = 1) can be obtained as

This is called the Bohr radius, represented by the symbol a0. Thus,
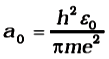
Substitution of values of h, m, ε0 and e gives a0 = 5.29 × 10–11 m. From Eq. (5), it can also be seen that the radii of the orbits increase as n2. The total energy of the electron in the stationary states of the hydrogen atom can be obtained by substituting the value of orbital radius in Eq. (4) as
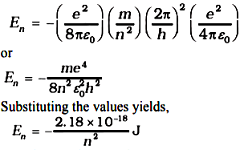
Atomic energies are often expressed in electron volts (eV) rather than joules. Since 1 eV = 1.6 × 10–19 J, Eq. (8) can be rewritten as

The negative sign of the total energy of an electron moving in an orbit means that the electron is bound with the nucleus. Energy will thus be required to remove the electron from the hydrogen atom to a distance infinitely far away from its nucleus (or proton in hydrogen atom).
The derivation of above Eqs. involves the assumption that the electronic orbits are circular, though orbits under inverse square force are, in general elliptical. (Planets move in elliptical orbits under the inverse square gravitational force of the sun.)