4 g of He gas is taken in a cyclic process as shown on the P-T diagram. The minimum temperature is 300 K and the maximum is 600 K. The pressures of the points are, PA = 1 × 105 N/m2 , PB = PD = 2 × 105 N/m2 .
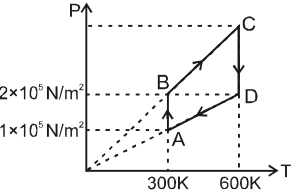
Net work done by the gas in the cycle is
(A) +ve
(B) – ve
(C) Zero
(D) +ve or –ve depending upon the value of pressure of state C.