(a) As pH = 7, pOH = 14 – 7 = 7 (at 25°C), pKb = –log Kb = – log (8.8 × 10–8) = 7.0555
Applying

Suppose V1 ml of HCl is mixed with V2 ml of imidazole (base) to make the buffer.
millimole of HCl = 0.02 V1
millimole of imidazole = 0.02 V2
As the buffer is of the base and its salt, 0.02 millimole of HCl will combine with 0.02 millimole of base to give 0.02 millimole of salt.
∴ millimole of salt = millimole of HCl
= 0.02 V1
and m.m. of base left = 0.02 V2 – 0.02 V1
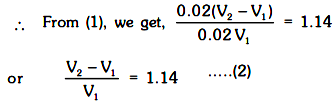
Given that V1 + V2 = 100 mL .....(3)
From (2) and (3) we get, V1 = 31.84 mL and V2 = 68.15 mL
(b) pH shall remain the same on dilution as both Kb and [salt]/[base] will not change.