Correct option: (c) 2 : 1
Explanation:
HCl will convert equivalent amount of NH4OH into NH4Cl and thus resultant mixture is a buffer.
Let HCl = x mL NH4Cl formed = x milli mol
NH4OH = (300 – x) mL, NH4OH left = (300 – 2x) milli mol
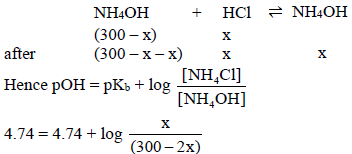
x = 100 mL = volume of HCl
200 mL = volume of NH4OH